
Side-effects are caused by the release of chemicals in the body which signal to the immune system it is time to mount a response. They may be uncomfortable, but can also be a sign the vaccine is working as intended. Side-effects are a sign the vaccine is prompting your body to mount an immune response. The Johnson & Johnson vaccine requires only one dose, has a generally lower rate of side-effects. More than 6.8m doses has been administered nationally when the pause was announced. If a link with the J&J vaccine is established, these cases, while serious, would suggest an exceedingly rare side-effect. On 13 April, the FDA and CDC recommended that states pause the administration of the Johnson & Johnson vaccine after reports that six women developed rare and severe blood clots, similar to those seen in a small number of people who have received the AstraZeneca vaccine elsewhere in the world. While this efficacy rate is lower than the two previously discussed vaccines, it still gives near perfect protection against hospitalization and death, and provides advantages in fighting the pandemic.

The FDA found this vaccine is more than 66% effective at preventing moderate to severe Covid-19. Importantly, this included South Africa, where the vaccine was found to be slightly less effective against the B1351 variant.
Moderna side effect trial#
Johnson & Johnson’s trial included more than 40,000 people across 19 geographic regions. The most recent vaccine authorized in the US is from the Johnson & Johnson subsidiary Janssen. On average, these symptoms cleared up within three days, and often less. We used results from the vaccine’s trials to describe how likely it is for people aged 18 to 64 to experience a given side-effect within one week of a dose of the vaccine. Among those trial participants, 15,168 people received the vaccine and the rest received a placebo. Moderna vaccine side-effectsįormally called mRNA-1273, this vaccine was developed by Moderna in partnership with the Niaid, but most people simply know it as the Moderna vaccine, which is a two-dose regimen spaced 28 days apart.Ī clinical trial involving more than 30,000 participants across 99 sites in the US found the vaccine was safe and effective, and protected people against Covid 94.1% of the time. This helps researchers understand the background rate of these side-effects in the population. People involved in the trials did not know whether or not they received the vaccine. One of the key numbers included in the graphs below is the rate of people who experienced side-effects after receiving a “placebo”, or an injection of saline instead of the vaccine. These ongoing studies can help identify the rarest of side-effects, and pinpoint people who may have special sensitivities to the vaccine, such as a potential for an allergic reaction. That monitoring goes hand-in-hand with reporting through several vaccine safety registries. These are sometimes referred to as phase IV trials.

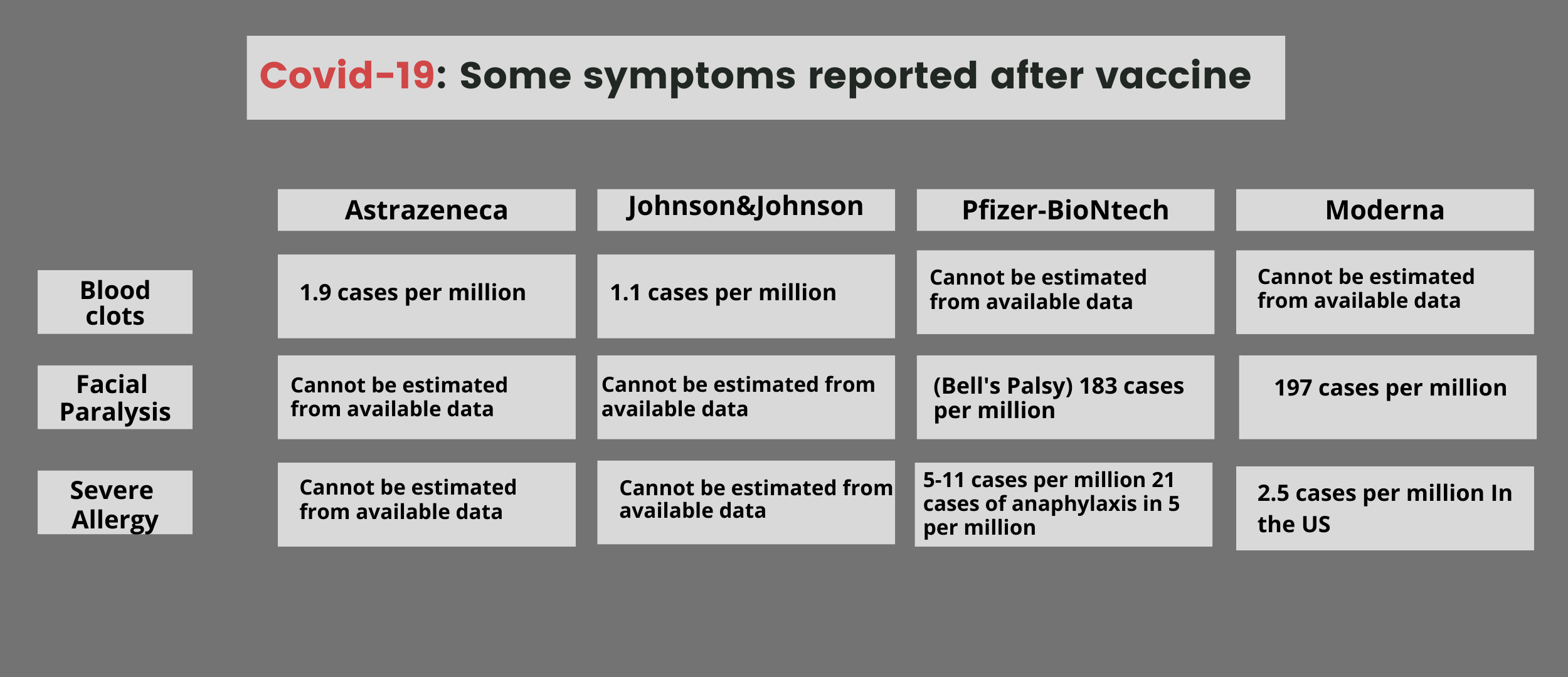
Their safety is tested in large trials of tens of thousands of people, then the FDA and the Centers for Disease Control and Prevention (CDC) continue to monitor vaccine safety data, including side-effects, after the vaccines are authorized. The vast majority of symptoms cause discomfort, but not a total disruption of your daily habits. Less common side-effects can also include nausea, chills and fever. For all the vaccines, the most common side-effects include:


 0 kommentar(er)
0 kommentar(er)
